History of fat analysis
Like protein and fibre determination, total fat determination is an important part of nutritional value analysis and quality assessment of food and animal feed, as fats are among the most important energy suppliers for humans and animals. However, the determination of the fat content does not only play an important role in the food and feed industry; the fat content in raw materials is also determined in the processing industry to assess the suitability for certain processing procedures and as part of quality control. Even in the environmental sector, the fat content in water and wastewater is determined to assess water purity.
However, a large proportion of the fats and fat-accompanying substances that need to be analysed are chemically and physically bound. Therefore, a digestion of the sample is necessary for a total fat determination. Over the course of time, various classic wet chemical methods have been developed for this purpose. And these methods have an astonishing longevity. Although modern rapid determination methods such as NIR (Near Infrared Spectroscopy) or NMR (Nuclear Magnetic Resonance) are now also an option, the wet chemical methods are still the reference methods in fat analysis.
Overview of the historical development of wet chemical fat analysis
| Digestion | Extraction | Evaluation | Name | Introduction |
|---|---|---|---|---|
| None | Solid-liquid extraction | Gravimetric | Soxhlet | 1879 |
| Hydrochloric acid hydrolysis | Solid-liquid extraction | Gravimetric | Weibull-Stoldt | 1892 |
| Alkaline hydrolysis (ammonia) | Solid-liquid extraction | Gravimetric | Röse-Gottlieb (Mojonnier) | 1893 |
| Sulphuric acid hydrolysis | Solid-liquid extraction | Gravimetric | Gerber | 1938 |
In general, a distinction can be made between reference methods, chemical rapid methods and spectroscopic rapid or secondary methods for the determination of total or crude fat. However, the classical gravimetric and wet-chemical methods are considered reference methods.
The gravimetric and wet-chemical methods all fall into the category of solid-liquid extractions:
Solid-liquid extraction
In solid-liquid extraction, a substance or group of substances is specifically isolated from a solid mixture using a liquid solvent. The classic hot extraction, in which the sample is transferred into a cellulose sleeve and then positioned in boiling solvent, was the first extraction of this kind. However, complete extraction of the sample is usually not possible with hot extraction because of the equilibrium between solvent and sample. Since the regulations and norms prevailing in the food and feed industry became stricter and stricter over time, the interest in more precise and effective methods for fat extraction grew steadily. Therefore, the classic hot extraction has been developed further and further, which in turn has led to the emergence of new solid-liquid extractions. The three best-known solid-liquid extractions are
- Soxhlet extraction,
- Butt and Twisselmann extraction and
- Randall extraction.
Soxhlet extraction
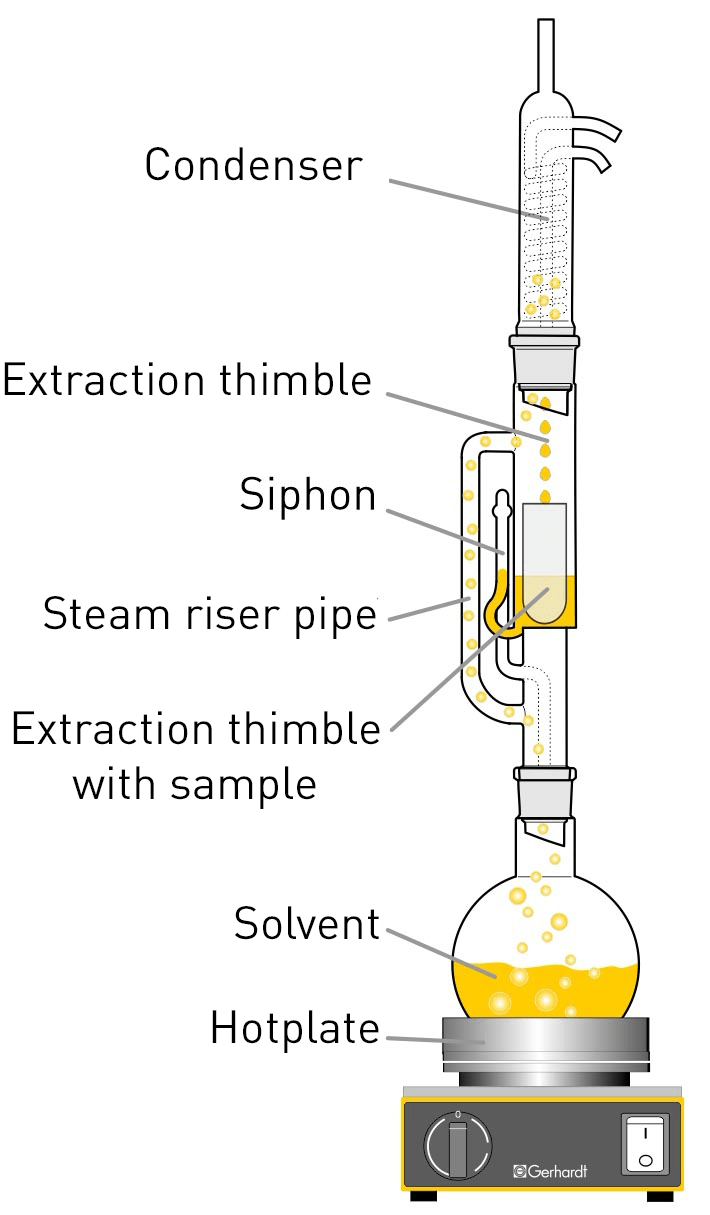
In 1879, Franz von Soxhlet developed a glass apparatus for continuous fat extraction. This so-called Soxhlet apparatus enables several extraction cycles to be carried out automatically with one sample.
The solvent is heated up to the boiling point in a flask, whereupon the solvent vapour rises via a side pipe into the condenser. In there, the vapour condenses and then drips into the extraction chamber. This chamber contains the sample to be extracted in a cellulose sleeve. The solvent that drops down fills the extraction chamber up to the height of the trap bend. Next, the suction siphon effect causes the solvent with dissolved extract to flow into the lower flask. By continuously repeating this process, a quantitative extraction can ultimately be achieved. After extraction, the solvent then has to be separated out in a separate apparatus and the isolated fat is dried for weighing.
Compared to classic hot extraction, in which the extraction of the sample takes place directly in boiling solvent, the temperature in the extraction chamber is lower, which makes the extraction process in the Soxhlet apparatus significantly more time-consuming. The time required for a quantitative Soxhlet extraction depends on the sample to be extracted and can take up to 8 hours or even more.
And although there are nowadays much more rapid extraction methods, in which the extraction of the sample then takes place again directly in boiling solvent, the Soxhlet method is still the most frequently described method for fat determination in feed and food.
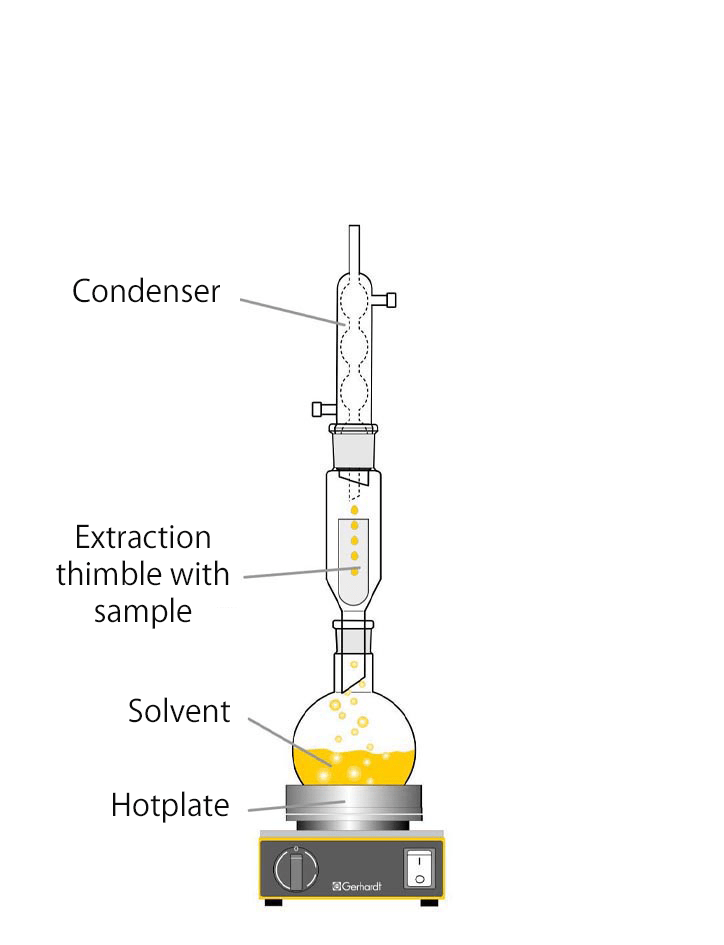
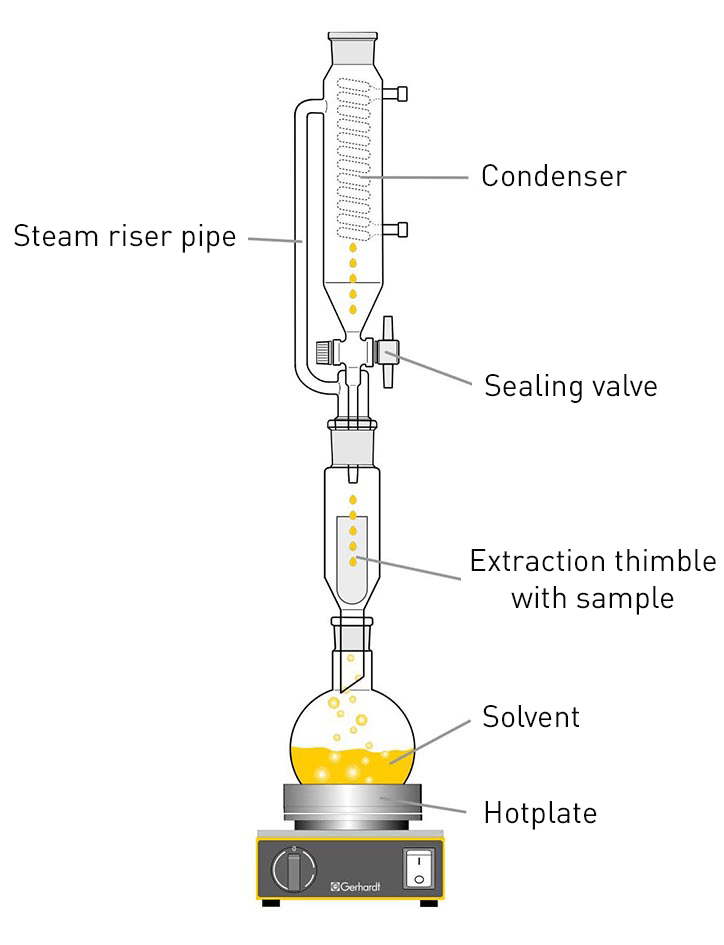
In Butt extraction, the extraction thimble with the sample is located above the heated solvent, which puts the sample into direct contact with the hot solvent vapours. The vapours rise to the condenser, where they condense and flow back through the sample into the flask. In the process, the sample is continuously extracted by the vapours and the solvent that drips back. Due to the continuous extraction process at higher temperatures, the sample can usually be extracted completely within a few hours.
After the extraction is complete, the solvent is removed from the extracted fat by distillation followed by vacuum drying and the extracted fat is weighed. The Twisselmann extractor (right) - a further development of the Butt tube - also has a shut-off valve to separate the solvent between the sample vessel and the condenser. After the extraction is complete, the shut-off valve can be closed and the solvent recovered.
Randall extraction
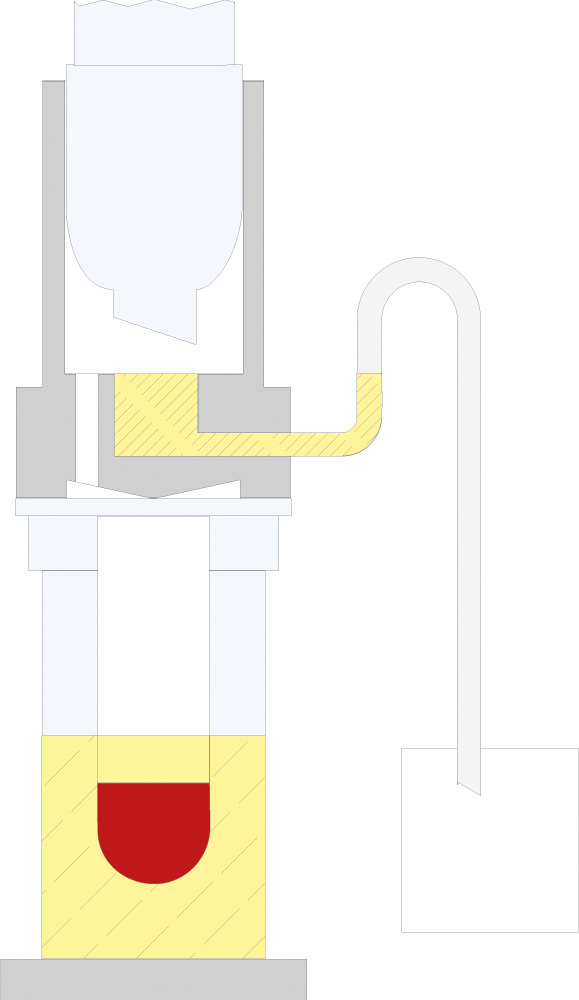
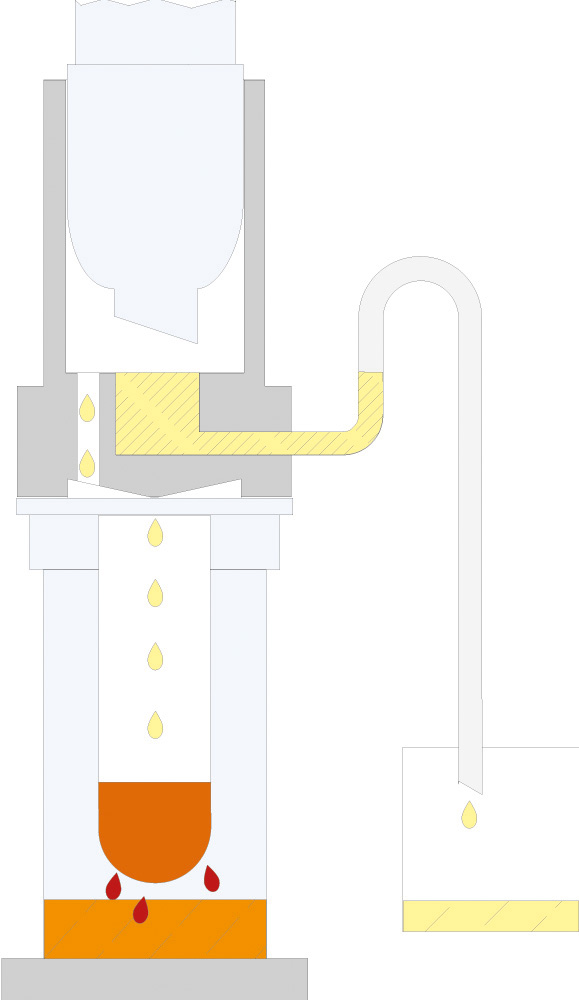
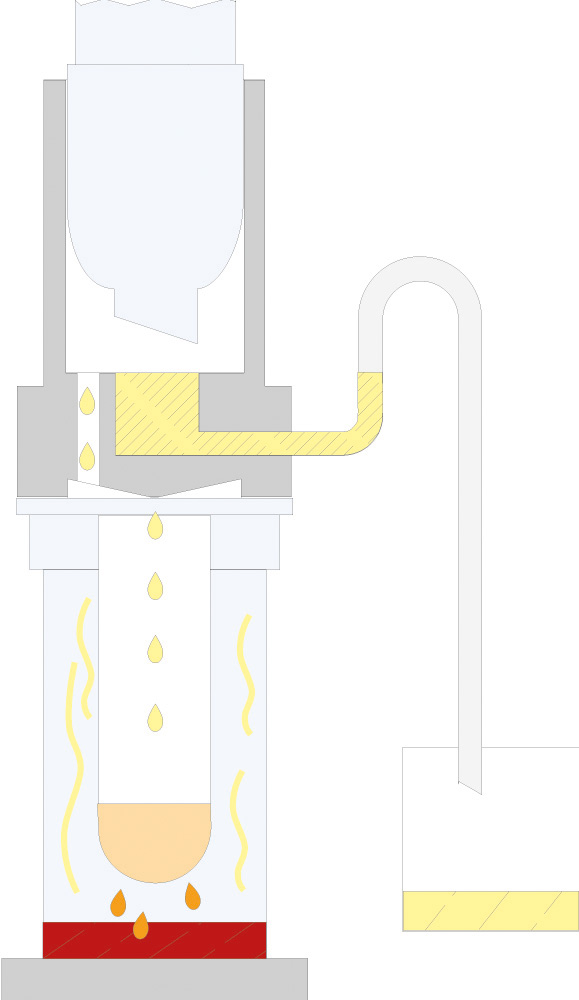
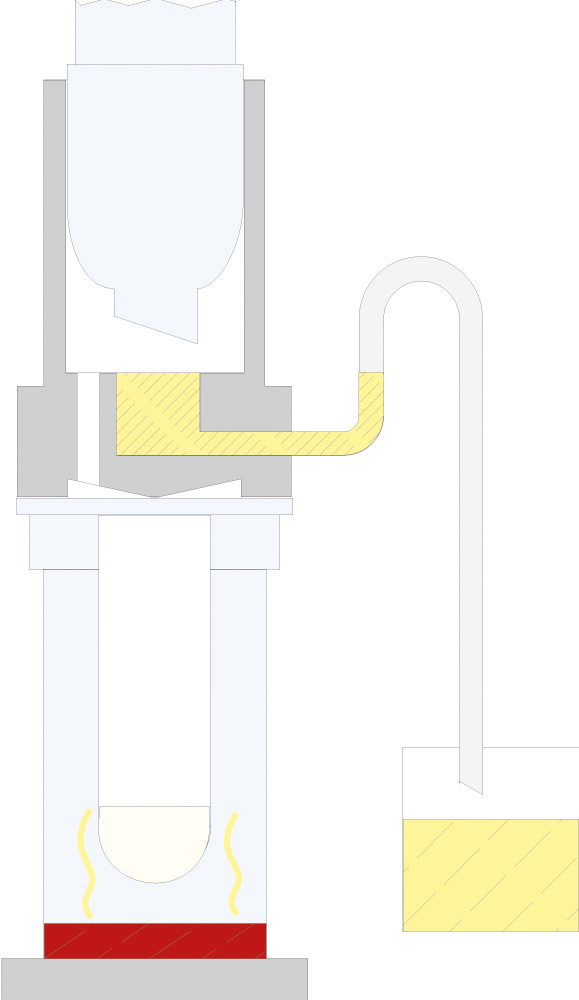
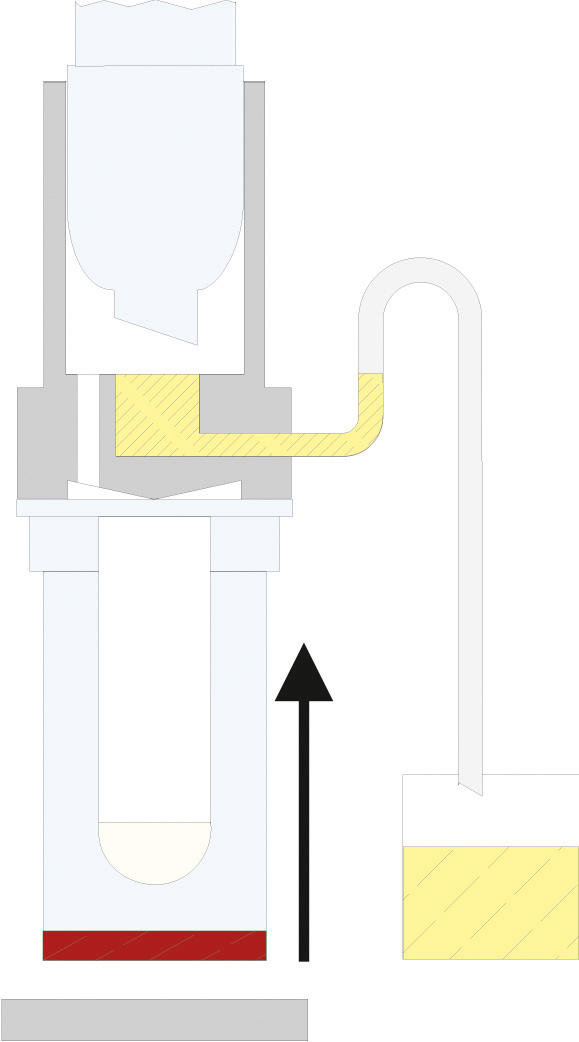
The principle of Randall extraction combines the speed of hot extraction with the efficiency of Twisselmann or Butt extraction. This principle is also used in the SOXTHERM and is shown schematically in the following figure:
In the first step, the extraction thimble containing the sample is positioned directly in the boiling solvent (1). This allows a large part of the fat to be extracted very rapidly - usually in 30 minutes. During the second step, the solvent level is lowered so that the sleeve with sample is no longer immersed in the liquid solvent (2). This prevents an equilibrium of dissolved fat between sample and solvent. In the third step, the solvent is extracted in hot solvent vapour and by the solvent dripping back down (3). The duration of this second extraction phase depends strongly on the sample type, but usually takes about 1 hour. After completion of the second extraction phase, the solvent is further evaporated and recovered (4). By recovering the solvent, it can be reused, which not only saves resources but also costs. In a final phase, the extraction beakers are being dried gently (5). The entire extraction process usually takes about 2 hours, so it is much more rapid than the classic Soxhlet method.
Fluid-fluid extraction
The principle of liquid-liquid extraction is based on different solubilities of two immiscible solvents. Usually, a polar solvent (e.g. diethyl ether) and a non-polar solvent (e.g. petroleum ether) are used for this. The extraction of the sample then works according to the principle "like dissolves in like": The polar components from the sample thus dissolve in the polar solvent, while the non-polar components dissolve in the non-polar solvent. Examples of apparatus-based conversions are the separatory funnel or the Mojonnier flask.
SFE - Supercritical Fluid Extraction
In supercritical fluid extraction (SFE), the extracting agent is separated from the sample matrix using supercritical fluids as extraction solvent. The extraction is usually done from a solid sample matrix, but can also be done from liquid samples. SFE can be used both as a sample preparation step for analytical purposes and on a larger scale. It is applied either to remove unwanted substances from a product, such as decaffeinating coffee, or to obtain a desired product, such as extracting essential oils.
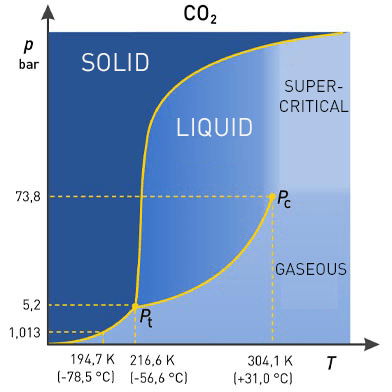
Carbon dioxide (CO2) is the most commonly used supercritical liquid, sometimes modified with Co solvents such as ethanol or methanol. The extraction conditions for supercritical carbon dioxide are above the critical temperature of 31 °C and the critical pressure of 74 bar. Under these conditions, CO2 behaves like a liquid and can therefore be used as a solvent to dissolve soluble components out of the sample matrix. After this process, the extract is returned to normal conditions, at which point the CO2 is volatilised and removed.


![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_hydrotherm_450x450px.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_soxtherm_6_450x450px.jpg)