Insects as an alternative source of protein
Insects are the alternative protein source of the future. On the one hand, because they have a good nutrient composition in terms of amino acids, minerals and fatty acids. On the other hand, they require hardly any water and space in cultivation, so they emit relatively little CO2.
As the main component of their exoskeleton, insects contain the polysaccharide chitin, which in turn contains nitrogen. In protein determination, this nitrogen contained in the chitin is recorded as crude protein, which increases the total value of the latter. However, since the nitrogen from the chitin cannot be processed by humans and animals, it should be considered separately.
In order to determine the chitin content separately, C. Gerhardt, together with the Research Institute of Feed Technology (IFF), has developed a cost-efficient and convenient analysis technique based on classical chemical methods. Namely, on the crude fibre and nitrogen determination with FIBRETHERM, KJELDATHERM and VAPODEST.
Chitin determination in food
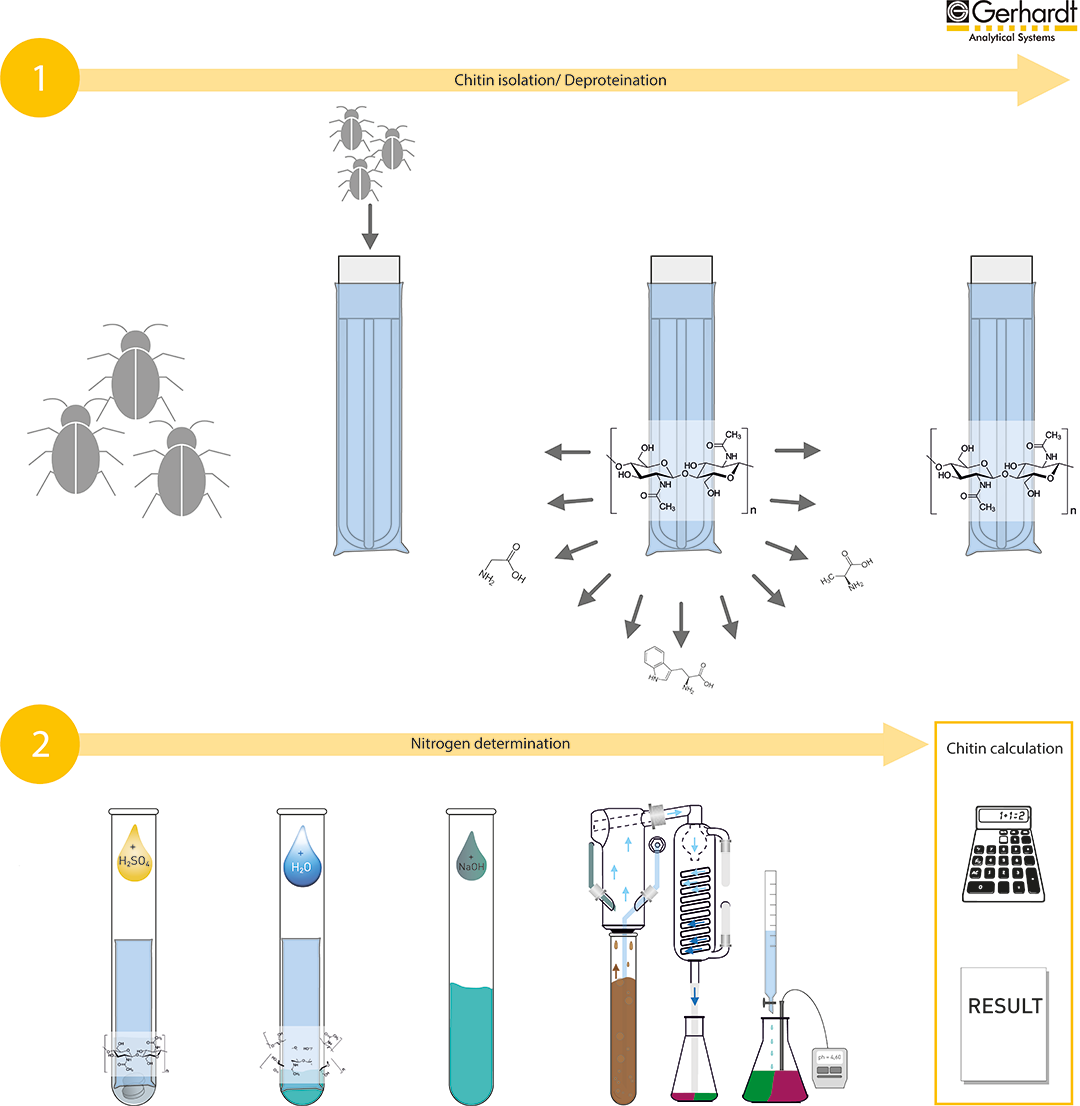
Sample preparation and weighing:
For the subsequent analysis, the samples must be homogeneous. Fresh insects should be dried. Insects with a high fat content can be frozen beforehand to avoid sticking during homogenisation. After sample preparation, approximately 1 g per sample including glass spacer and FibreBag is weighed in.
- App note: If the fat content of the sample is above 10%, it is recommended to degrease the sample.
Alkaline hydrolysis and drying:
The samples are placed in the carousel, then the carousel is placed in the FIBRETHERM and the method is started. Once the FIBRETHERM method is complete, the glass spacers and FibreBags are placed back into the crucibles. The glass spacers are rinsed with distilled water to remove any residue in the FibreBags. The crucibles with the FibreBags are dried at 103°C for 4 hours or overnight.
Nitrogen determination acc. to Kjeldahl
Addition of the chemicals:
Each FibreBag is placed in a digestion tube. Then the appropriate chemicals are added for digestion.
Digestion:
The samples are digested in the KJELDATHERM or TURBOTHERM with the specified parameters.
Distillation:
After cooling the samples, a steam distillation is carried out with the VAPODEST:
- App note: When using our KJELCATS, it is important to observe a colour change from blue to brown after adding NaOH, indicating that the NaOH has been added in excess.
Titration:
Add 3-4 drops of the indicator solution to the receiver solution and titrate with the standard solution until the colour changes from green to violet. If you determine the endpoint with a pH electrode, you do not need to add the indicator solution.
Calculation:
The chitin content can be calculated with the following equation:
ꞷchitin = Chitin content [%]
V1 = Volume of standard solution used for the residue after deproteination [ml]
V0 = Volume of standard solution used fort he process blank [ml]
ceq;soll = Equivalent concentration of the standard solution
t = Titre oft he standard solution
20,319 = Factor for recalculating the chitin content
msample = Weight of the sample put into the FibreBag [g]
Example results Chitin content in insects
| Dried yellow mealworm larvae (Chitin content in %) | Mealworm press cake (Chitin content in %) | Dried crickets (Chitin content in %) | |
|---|---|---|---|
| Sample 1 | 6.163 | 9.664 | 9.553 |
| Sample 2 | 6.157 | 9.777 | 9.553 |
| Sample 3 | 6.224 | 9.714 | 9.599 |
| Sample 4 | 6.045 | 9.711 | 9.662 |
| Sample 5 | 5.982 | 9.650 | 9.633 |
| Mean value chitin content [%] | 6.114 | 9.703 | 9.596 |
| Standard deviation chitin content [%] | 0.098 | 0.050 | 0.054 |


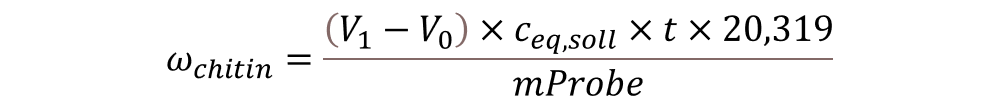
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_fibretherm_450x450px.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_kjeldatherm_450x450px.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_vapodest_450x450px.jpg)