In 1883, Johan Kjeldahl introduced his "New Method for the Determination of Nitrogen in Organic Bodies", revolutionising nitrogen analysis and setting new standards. Since then, the method has become indispensable in areas such as food analysis, feed analysis, soil analysis and water analysis. However, it is by no means limited to these areas: This versatile method can also be found in the industrial or in the pharmaceutical sectors and wherever the nitrogen content is important.
Thanks to the wide range of possible applications, the high precision and the simple execution, the Kjeldahl analysis is still considered to be the reference method today. Because the Kjeldahl analysis can be used to determine all nitrogen components. In addition to the total nitrogen content, individual components such as ammonium, nitrate, nitrite and organically bound nitrogen can be determined from a wide variety of sample matrices.
The particular advantage is the versatility of the matrices to be analysed. For example, in addition to cereals, animal feed, dairy products or other foodstuffs, slurry, sewage sludge, composts and soils as well as aqueous extracts and waste water can also be analysed for their nitrogen content. Especially with highly inhomogeneous sample material, there is hardly any alternative to Kjeldahl because of the high sample weights.
The Kjeldahl analysis for nitrogen and protein
In the traditional application, manual laboratory heaters are used, as well as round-bottom flasks for digestion and Erlenmeyer flasks for distillation. After the publication of the Kjeldahl method, C. Gerhardt set itself the goal of optimising this classic application. Over the last decades, a large number of different instrument types have been developed for this purpose. It all started with large cast-iron racks; today there are highly precise block digestion units and steam distillation units with result calculation and automatic sample feeding.
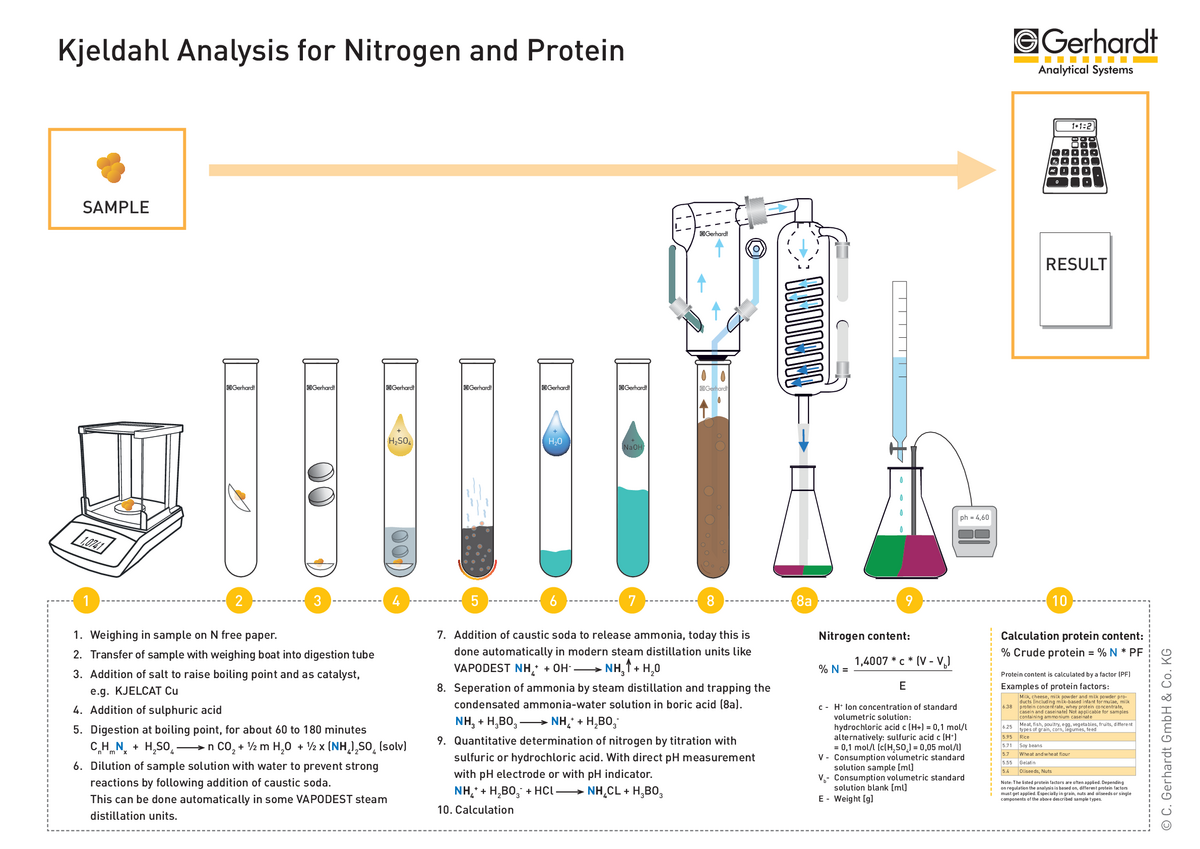
Essentially, however, the Kjeldahl analysis can still be divided into the 3 work steps that also form the basis of the classical application:
- Digestion of the samples with sulphuric acid
- Distillation of the digestion solution with steam
- Titration of the distillate and calculation of the result
The chemistry has therefore changed only slightly, but the overall process is now fully automated and adapted to the conditions of modern laboratories. The use of a digestion block and steam distillation system with integrated titration has many advantages for the user.
The daily work of the laboratory staff is much safer, as the time spent at the equipment has been reduced and it is equipped with various safety features. In addition, the sample throughput has been significantly increased by the automated processes. At the same time, the safety of the analysis is also increased.
For a better understanding of how Kjeldahl analysis works with automated analysis systems, a diagram explaining the process step by step is given below:
Total Kjeldahl Nitrogen vs. Total Nitrogen
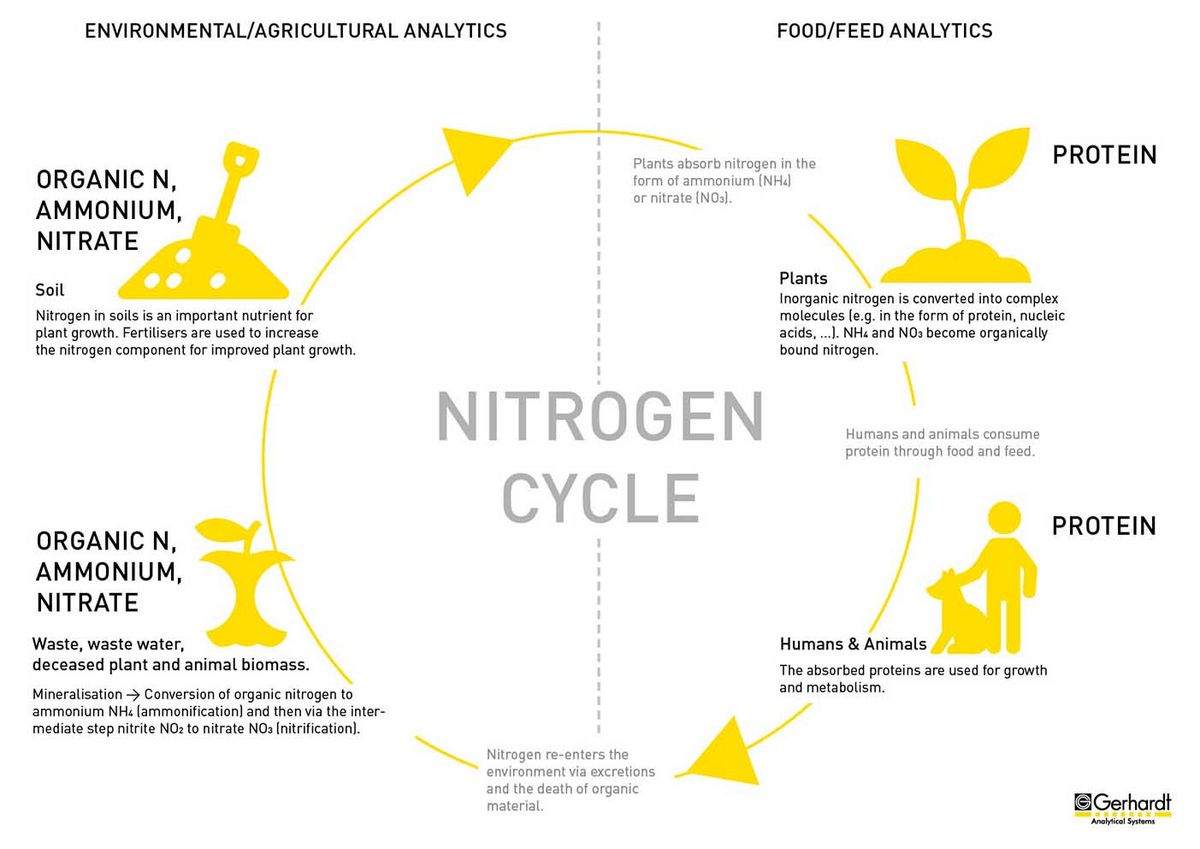
The two terms Kjeldahl nitrogen (Total Kjeldahl Nitrogen, TKN) and total nitrogen (Total N) are often confused or even considered to be the same, but they represent different values when determining the nitrogen content.
The different names often lead to the assumption that TKN and Total N are two different forms of a nitrogen compound. In fact, however, the two terms describe different sum parameters in nitrogen analysis.
While TKN describes the proportion of total bound organic nitrogen and ammonium (NH4) in a sample, Total N additionally includes nitrite (NO2) and nitrate (NO3).
The term Total Kjeldahl Nitrogen (TKN) or Kjeldahl nitrogen is therefore not derived from a specific nitrogen compound, but from the analytical method used: in other words, the Kjeldahl method.
Since the Kjeldahl method can be used to determine not only Kjeldahl nitrogen but also ammonium nitrogen (NH4), nitrite nitrogen (NO2) and nitrate nitrogen (NO3), it is also possible to determine the two sum parameters TKN and total N in addition to the individual nitrogen compounds. The different values are relevant for different analytical areas:
Total Kjeldahl Nitrogen (TKN)
Total Kjeldahl Nitrogen (TKN)
Kjeldahl nitrogen is particularly relevant in the context of wastewater treatment, as the determination of TKN is mandatory in many international regulations. During the individual process steps, e.g. in the context of biological wastewater treatment, the parameter is constantly monitored. This means that the entire process can always be monitored for quality and adjusted if necessary. The protein content can also be calculated from the Kjeldahl nitrogen contained in the sample (see Protein section).
Ammonium nitrogen
The nitrogen that enters the environment via excretions and the decay of organic material is initially present mainly in the form of organically bound nitrogen. During mineralisation, this is converted into ammonium (NH4) in the first step. In technical terms, this process is also called "ammonification". Subsequently, ammonium is converted to nitrate (NO3) via the intermediate step nitrite (NO2), this is called nitrification.
Mineralisation is a natural process that takes place in the soil when nitrogen is extracted from complex organic compounds that are not available to plants and converted into a mineral nitrogen species.
However, this process is also used in environmental analysis, for example in sewage treatment plants when treating waste water, as the water is purified in this way. In drinking water, for example, the ammonium value provides information about the degree of pollution of the water.
Ammonium is also relevant for agriculture, as the nitrogen in this compound can be absorbed and processed by plants. Since it has a positive effect on the growth of plants in fields, agricultural areas are treated with fertilisers. Quickly available ammonium nitrogen is thus added to the plants.
Nitrate nitrogen.
Nitrate nitrogen
Similar to ammonium nitrogen (NH4), nitrate nitrogen (NO3) can also be absorbed particularly well by plants and promotes their growth. Nitrate-based fertilisers are used in modern agriculture for more efficient use of available agricultural land.
However, since nitrate is only harmless to health up to certain amounts, there are strict limits worldwide for drinking water, for example. Excess nitrate can enter groundwater and other bodies of water through excessive fertilisation of agricultural land. Therefore, in areas with intensive agriculture, compliance with the limit values must be checked regularly.
Protein (calculated with the protein factor)
The determination of protein content is particularly relevant for food and feed. Organically bound nitrogen (e.g. in amino acids, proteins, nucleic acids (DNA), ...) plays a central role in the structure and metabolism of living organisms.
Since in most foods it can be assumed that the Kjeldahl nitrogen mainly derives from the proteins, the nitrogen content of a sample is directly related to the protein content. For most samples, the nitrogen content in the proteins is 16 %, therefore a conversion factor of 6.25 (nitrogen content [%] * protein factor = protein content [%]) results. However, for certain samples the proportion differs, resulting in a different protein factor (see table below).
Protein factor (examples)
| Milk | 6,25 |
| Meat | 6,25 |
| Cereals (except wheat) | 6,25 |
| Wheat | 5,7 |



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/3/2/csm_cg_publications_800x800_starch_a16348c882.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/7/csm_kachel_800x800p_methode_dumas_blanco_web_ac96dce359.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_kjeldatherm_450x450px.jpg)
![[Translate to English:] [Translate to English:]](/fileadmin/Redaktion/Freigestellte_Produktbilder/web_pic_vapodest_500_kachel_450x450px.jpg)