Kjeldahl vs. Dumas in nitrogen and protein analysis
The classical Kjeldahl method has been recognised as the reference method for nitrogen determination since its publication in 1883 and was considered unchallenged for a long time. The much older combustion method according to Dumas from 1831 has only been able to establish itself as the reference method for nitrogen determination in recent decades. Thanks to the much more easily reproducible framework conditions for the Dumas analysis today, however, it is steadily gaining in popularity.
Due to the different approaches, both methods have their specific advantages and disadvantages. Deciding which method is the right choice for a laboratory depends on various factors such as the laboratory environment, the laboratory staff and the sample matrix.
In any case, it is important to analyse the individual situation of a laboratory and then consider the advantages and disadvantages of the two methods.
Dumas method - ideal for the high-throughput laboratories
One of the main reasons why the Dumas method is nowadays recognised in so many standards and is gaining more acceptance is that it is safer for the operator. In contrast to the Kjeldahl method, the Dumas method does not require the use of harmful or toxic chemicals or catalysts. This is beneficial for the operator and the laboratory environment as well as for the global environment.
In addition to the toxic chemicals used, harmful fumes are produced during the Kjeldahl analysis. These can also pose a danger to the operator if not properly vented.
Which brings us to the next difference between Dumas and Kjeldahl: Analytical systems that work according to the Dumas method - in contrast to Kjeldahl apparatuses - do not require space in the fume hood. And since the space inside of a fume hood is usually limited and thus expensive, this is another advantage of the Dumas method.
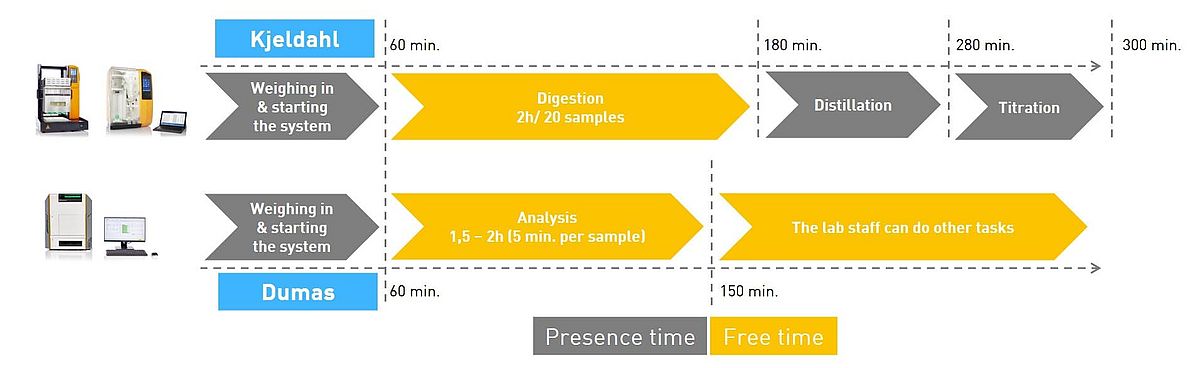
Moreover, in a society where time is becoming more and more expensive and efficiency more and more important, the automated Dumas method has the enormous advantage that the analysis time is far shorter than with Kjeldahl.
Although the modern Kjeldahl apparatuses are also highly optimised and the analysis is largely automated, the analytical processes simply take much longer. While the operator gets a result within a few minutes with combustion analysis, it can take up to several hours with Kjeldahl.
The presence time spent with the analytic system is also lower with Dumas than with Kjeldahl. As the entire Dumas process is fully automated and takes place within one single analytical system, for the Kjeldahl analysis two systems are necessary- one for digestion and one for distillation. Due to the reduced analysis and presence time, laboratories can save time and thus also costs per sample with the help of the Dumas method.
This is an important factor especially for laboratories with a high sample throughput. However, with the Dumas method the sample matrix should not be varied too often, as this can lead to contamination of the samples, which can falsify the analysis results.
Analysis systems using the Dumas method are therefore well suited for laboratories that regularly analyse a specific sample matrix and have a high sample throughput. It is optimal if the analysis system is continuously in operation, as switching it on and off is very time and energy consuming due to the extreme temperature conditions.
Kjeldahl method - the all-rounder in nitrogen analysis
Despite the advantages of Dumas, the Kjeldahl method is still the dominant reference method. It is the more practical solution especially in laboratories with lower sample throughput and changing sample matrices that are analysed according to a wide variety of applications.
Additionally, the flexibility in sample weight with Kjeldahl is an advantage that should not be underestimated. The Kjeldahl analysis can be carried out with a wide variety of laboratory glassware and with sample quantities of up to about 10 grams. With the Dumas method, on the other hand, a maximum of only 1 gram can be analysed.
Especially with inhomogeneous samples such as soil, the flexibility of the sample weight is a decisive advantage of the Kjeldahl method. This also applies to liquid samples. Although there are aids for Dumas apparatuses in the form of special absorbents, with which the analysis of liquid samples is possible, the purchase of consumables entails further costs per sample.
It is clear now, that both methods have strong advantages and that deciding which method suits a laboratory always requires a precise analysis of the current situation. As our team of experts is well versed in both methods, we can provide you with objective consultation on which method is suitable for your laboratory.