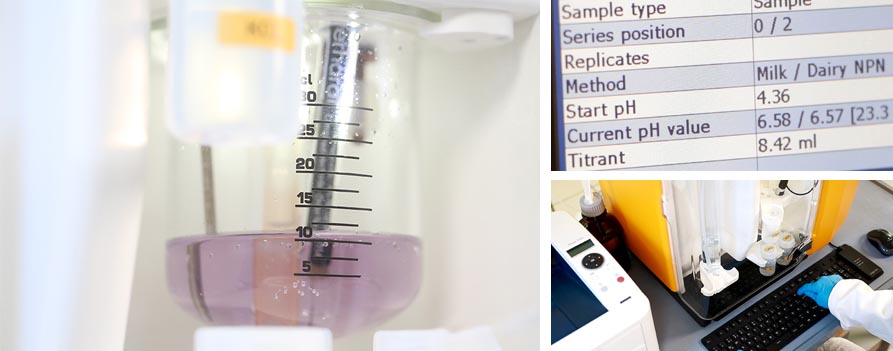
The most common titration methods in modern fully automatic distillation units are the pH determination via colorimetric detection and the pH measurement with a pH electrode based on the electrochemical measurement. Both methods are generally suitable for automatic systems. The measurement with the pH electrode however has the significant advantage that it monitors continuously the actual pH of the solution. Thus C. Gerhardt instruments like VAPODEST® 50s are using pH-electrodes for the titration. Combined with multiple other advantages it is the perfect method for fully automatic systems:
- accurate results - electrochemical measurement monitors the real pH in the titration solution + possible to display the pH graph
- highest possible reproducibility
- high flexibility - any concentration / amount of acid possible
Additionally the method eliminates the biggest limitation of the colorimetric detection:
- no sensitivity against light (e.g. illumination of the laboratory)
- no experience with the handling of indicator solution necessary
- no expensive indicator solution needed
- no adjustment of the receiver solution
C. Gerhardt pH measurement fullfils at minimum, the accuracy criteria specified recovery and repeatability by official national and international standards.
Certainly the pH measurement applied by C. Gerhardt is mentioned modern national and international Kjelddahl norms, e.g. ISO 8968-1:2014 (IDF 20-1:2014) - Milk and milk products - Determination of nitrogen content - Part 1: Kjeldahl principle and crude protein calculation.
As marketing and sales tool we have created a chart with a brief comparison of both methods and an additionally graphic to show the key difference between the pH measurement with a pH electrode and the colorimetric detection.